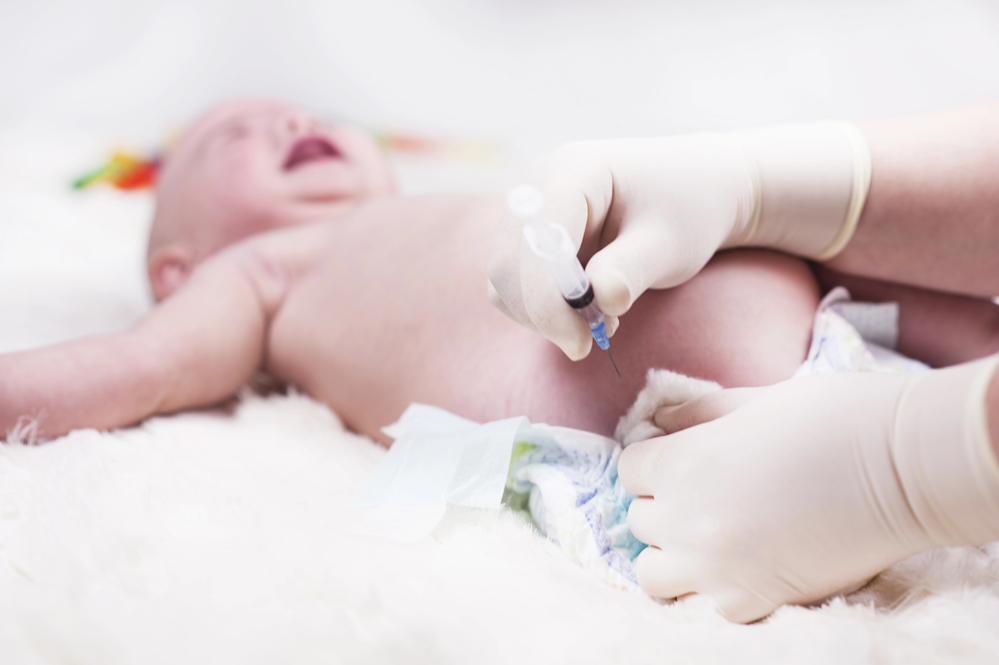
The DTaP vaccine is a combination vaccine on the childhood schedule recommended by the US Centers for Disease Control and Prevention (CDC). It is marketed as an injection that provides immunity against diphtheria, tetanus, and pertussis—the latter commonly known as “whooping cough.”
DTaP is routinely administered as a five-dose series, usually beginning at two months of age.
The components of this three-in-one vaccine include diphtheria and tetanus toxoids (inactivated toxins) and either whole cells or bits of the pertussis bacterium.
The various iterations of this combination vaccine are largely distinctions without a difference. The primary differences in this line of vaccines lie with the pertussis component of the vaccine. The little “a” means “acellular,” which is the predominant form used in today’s DTaP vaccines.
The shift from the whole cell pertussis vaccines to acellular vaccines began in the 1990s after multiple reports of side effects were associated with DTP vaccines. The “whole cell” pertussis bacterium originally used in DPT vaccines—and still widely used in developing countries—was considered too reactive. It has been replaced by what some consider the less-reactive acellular DTaP vaccine.
While our primary focus will be on the DTaP vaccine itself, the full story of this controversial vaccine cannot be understood without revisiting the history of whooping cough, diphtheria, and tetanus and the history of the original pertussis vaccine.
Pertussis—The Whooping Cough
“I was astonished and not a little perturbed to find that when you draw a graph of the death rate from whooping cough that starts in the mid nineteenth century, you can clearly see that at least 99 percent of the people who used to die of whooping cough in the nineteenth and early twentieth century had stopped dying before the vaccine against whooping cough was introduced, initially in the 1950s and universally in the 1960s. [Emphasis added.]
“I also realized that the reason the Department of Health’s graphs made the vaccine appear so effective was because they didn’t start until the 1940s when most of the improvements in health had already occurred, and this was before even antibiotics were generally available. If you selected only deaths in under-15-year-olds, the drop was even more dramatic — by the time whooping cough vaccine was part of the universal immunization schedule in the early 1960s all the hard work had been done.” [Emphasis added.] — Dr. Jayne Donegan
First recognized in the Middle Ages, pertussis, or whooping cough, has held many names over the centuries. The word “pertussis” was coined by English physician Thomas Sydenham in 1670 and came to mean “violent cough.”
In 1906, Jules Bordet and Octave Gengou invented a special medium, called Bordet-Gengou (BG) medium, which they claimed would allow for isolation and identification of the pathogen responsible for this chronic coughing condition.
BG medium was made from “a melted fluid consisting of a glycerin extract of potato, agar, and salt solution, to which an equal volume of defibrinated rabbit or human blood was added.”
Bordet and Gengou claimed their medium could promote the growth of bacterial colonies if inoculated with sputum taken on the first day of the disease. According to Bordet and Gengou this process would create the conditions for isolation of the alleged microbe.
History records that their first successfully isolated pertussis bacillus came from the expectorate of Bordet’s son who had just contracted a serious cough.
This “discovery” was published in the Annales de l’Institut Pasteur in 1906 under a paper titled, “Le microbe de la coqueluche“—”The cough microbe.” The alleged bacterium was subsequently named Bordetella pertussis, in honor of Bordet.
Once this microbe was identified as the specific agent leading to widespread respiratory ailments, the daily hazards of the turn-of-the-century industrial era—filthy street sewage, poor nutrition, heavy air pollution, and overall squalid living conditions—were no longer deemed causes for illness. Instead, they were sidelined by those who were in hot pursuit of the opportunity to invent a vaccine.
In 1914, the first whole-cell pertussis vaccine was licensed in the US. In the late 1940s, these injections were largely considered ineffective and were replaced by the diphtheria-tetanus-pertussis (DTP) vaccines.
In today’s medical literature pertussis is commonly characterized as a bacterial infection with symptoms similar to a common cold. Some cases develop into chronic rapid coughs accompanied by a distinctive high-pitched “whoop.”
Most pertussis cases occur in children under five years of age. Around 82% of pertussis cases in children are benign, and cases in adults are uncommon.
Fatal cases of pertussis in the United States are rare. Between 1900 and 1945, before widespread use of the pertussis vaccine began, the mortality rate of pertussis dropped by 92%, from 16.1 per 100,000 to 1.3 per 100,000. This reduction is attributed to advancements in living conditions, nutrition, and health care.
A 2020 study from the journal Vaccines estimated that, in the absence of mass vaccination, the risk of an infant younger than 1 year old contracting a fatal case of pertussis in the US in the 21st century is about 0.002%. The study also noted:
“CDC analyses of pertussis [absent mass vaccination] have shown that permanent disability in pertussis survivors was very rare.”
Diphtheria
“Today your child has about as much chance of contracting diphtheria as she does of being bitten by a cobra.” — Dr. Robert Mendelsohn, MD
Diphtheria is conventionally characterized as a rare bacterial disease caused by Corynebacterium diphtheriae. The name of the disease is derived from the Greek diphthera, meaning “leather hide”—a reference to the tough pseudomembrane that forms in the patient’s throat.
The bacteria Corynebacterium diphtheriae is said to have first been observed by Edwin Klebs in 1883 and cultivated by Friedrich Löffler in 1884.
But Löffler was only able to culture the organism from the nasopharyngeal cavity, not from the throat, leading him to posit that further damage from this disease must result from a soluble toxin.
In the early 1890s, Emil Von Behring and Shibasaburo Kitasato reported that serum taken from experimental animals who had been immunized against the diphtheria toxin could be used to prevent and treat diphtheria in humans.
Von Behring, considered by some to be the “father of immunology,” went on to develop the first diphtheria antitoxin and started the first human trial of serum therapy against diphtheria in 1892. He was awarded the first Nobel Prize for Physiology or Medicine in 1901 even though his initial serum formulation was unsuccessful due to poor serum quality.
Recent investigation reveals this legendary figure to have employed fundamental methodological tricks that apparently rigged the outcome of his experiments. A detailed analysis found that he was “pretreating” the lab animals by giving them therapies “designed to alleviate toxicity.”
This same exhaustive exposition of von Behring’s work observed that he was “pretreating his cultures and animals with iodine trichloride and zinc chloride and using these substances to ‘disinfect’ the toxins in the cultures, gradually ramping up the doses, and thus providing the illusion of “immunity.”
Von Behring openly admitted that “immunity was not permanent and that unfavorable conditions left the animals to the same disease as if they had not been immunized.” [Emphasis added.]
In 1896, the first indications that serum therapy might cause adverse events appeared when a healthy infant died from what was believed to be an anaphylactic reaction to the serum. In the child’s obituary, the father—a well-known pathologist—declared that his son’s death was “due to the injection of Behring’s serum for immunization.”
The poor-serum problem was purportedly resolved when Paul Ehrlich, considered the “father of chemotherapy,” developed standardized measuring techniques and used larger animals (horses) to derive diphtheria antitoxin. Later it was discovered that the horse-derived antitoxin was also associated with anaphylaxis and serum sickness.
The safety and efficacy of diphtheria antitoxin was not universally accepted.
In 1907, Dr. Charles Page asked the question, “Diphtheria: Is the Prevailing Antitoxin Treatment Only Another Medical Delusion?,” in the title to his article published in Medical Brief, A Monthly Journal of Scientific Medicine and Surgery. Page believed that improvements in sanitation would be a more effective remedy for diphtheria.
Page’s sentiment was echoed by Dr. James Cumming in an article published in the Journal of the American Medical Association in 1922.
Cumming pointed to the fact that mortality rates from diphtheria had decreased over the previous 30 years, before diphtheria toxoids were widely used.
He attributed this decrease to improvements in hygiene and sanitation:
“The eradication of diphtheria will not come through the serum treatment of patients, by the immunization of the well, or through the accurate clinical and laboratory diagnosis of the case and the carrier followed by quarantine; rather it will be attained through the mass sanitary protection of the populace subconsciously practiced by the people at all times.”(JAMA, 1922, p. 682.)
Nevertheless, despite the correlations between diphtheria and poverty, poor social conditions, poor nutrition, and poor sanitation—and despite the accompanying statistical decline of diphtheria in developed countries as those conditions improved—the march towards wholesale vaccination continued apace. Starting in the late 1940s, the diphtheria nostrum was incorporated with the tetanus toxoid, and the combination vaccine was routinely used by doctors to immunize patients against these three diseases.
Tetanus
“Tetanus vaccine is probably one of the most ridiculous vaccines ever. Your chances of getting tetanus are about the same as you walking outta here and getting hit by a meteor. If you get a cut or a puncture wound and you put peroxide on it, your chances of getting tetanus are zero because tetanus organism is anaerobic. It cannot live in oxygen. Tetanus comes from the bowels of animals. As long as you don’t have a sheep or a cow in your house, I don’t think you are in any danger.” — Dr. Russell Blaylock, MD
Tetanus is commonly characterized as “an acute disease caused by an exotoxin produced by the bacterium Clostridium tetani.” The signature features of tetanus are generalized rigidity in the body and convulsive spasms of skeletal muscles.
There are five kinds of tetanus: Subacute tetanus, Local tetanus (rare), Cephalic tetanus (very rare), Generalized tetanus (most common: about 80% of all cases) and Neonatal tetanus (eliminated from developed countries before either a vaccine or antibiotics were invented—primarily because of improvements in basic hygiene.
The alleged organism that causes these potentially fatal conditions is sensitive to heat and cannot survive in the presence of oxygen.
The conventional belief is that C. tetani enters the body through a wound, causing the spores to germinate in the presence of anaerobic conditions, which in turn cause toxins to be produced and disseminated via blood and lymphatics.
There are, however, no laboratory findings confirming this characterization. The diagnosis of tetanus is entirely clinical. It is also noted that “C. tetani is recovered from the wound in only 30% of cases andcan be isolated from patients who do not have tetanus.” [Emphasis added.]
Tetanus became a nationally reportable disease in the United States in 1947 even as there was a marked decrease in mortality from tetanus from the early 1900s to the late 1940s.
In a population of around 145 million, 472 tetanus cases (pre-vaccination) were reported in the entire US in 1947.
By the late 1940s, tetanus toxoid-containing vaccines were introduced into routine childhood vaccination schedules.
A Brief History of the Pertussis Vaccine
”When we look at the actual data, we see that although many people did die from whooping cough in the early part of the 1900s, by the time the vaccine had been introduced the death rate in the United States had declined by more than 90 percent. Using the source that was referenced to make the statement in the Pediatrics paper, we see that the decline in deaths from the peak was approximately 92 percent before the introduction of the DTP vaccine.” — Roman Bystrianyk and Suzanne Humphries, MD, co-authors of Dissolving Illusions: Disease Vaccines and the Forgotten History
The story of the pertussis vaccine is largely the story of bacteriologist Pearl Kendrick and public health scientist Grace Eldering.
Though many pharmaceutical companies in the United States were offering pertussis and mixed-serum pertussis vaccines in the early 1900s, none proved to be effective.
In 1931, the American Medical Association’s Council on Pharmacy and Chemistry found no “evidence even for the presumptive value of stock or commercial vaccines” because “the pertussis vaccines seem to have absolutely no influence [as a preventive], and after the disease is thoroughly established even freshly prepared vaccines seem useless.”
In 1932, Kendrick and Eldering began the whooping cough research project in Grand Rapids, Michigan. They alleged that they had improved the methods used for growing the pertussis bacillus, which allowed them to design and direct the first large-scale controlled clinical trial for the pertussis vaccine. This was hailed at the time as one of the greatest field tests in microbe-hunting history. Keep in mind that in the 1930s, there were no accepted standards and few established models for conducting field studies.
The field trial ran from 1934 to 1937 and was composed of 5,815 children. The vaccinated group was made up of “children of acceptable age and history who presented themselves at the city immunization clinics for pertussis vaccination.”
The control group was “selected at random from a list of non-immunized children maintained by the Grand Rapids City Health Department.”
Even though an approximately equal sample of children of the same age comprised both groups, the original field trial design was methodologically flawed. The “vaccinated” experimental group was self-selected, but the unvaccinated control subjects were randomly chosen. In addition to this procedural defect, 1,603 observations (28%) from the study’s early years were not included in the final analysis.
Along with these operational deficiencies was the largely overlooked fact that the study was conducted during the height of the Great Depression (an era of extreme deprivation in which daily life consisted of grinding poverty, food scarcity, substandard housing, and extraordinary social stressors). As Grace Elder noted, “[W]e learned about pertussis and the Depression at the same time.”
In the summer of 1936, America’s then-premier epidemiologist, Wade Hampton Frost, a professor of epidemiology at Johns Hopkins University, was tasked with reviewing the Kendrick-Elder study. He identified four major problems with the study.
Due to the long, slow build-up of the trial, he noted, the study population overall was quite heterogeneous, which meant that:
- In the early years of the trial, follow-up of control children was either inadequate or the records were incomplete;
- Recruitment to the trial varied over the life of the study, as did the frequency of nursing visits to look for whooping cough;
- The possibility of unknown differences between experimental and control groups existed because of differences in the way they had been recruited.
- There was a question as to whether the rates of other communicable diseases were also lower in the experimental group, as might be expected, if the vaccinated children were from a higher socioeconomic group than were children in the control groups.
Nevertheless, the field trials were deemed a success, and Michigan began distributing the pertussis vaccines in 1940.
By 1942, Kendrick and colleagues combined the pertussis vaccine with diphtheria and tetanus toxoids to produce the diphtheria-tetanus-pertussis (DTP, also known as DPT) combination vaccine, which would lay the groundwork for future vaccine developments.
In 1947, this combined vaccine was recommended for use by the American Academy of Pediatrics. In 1948, it was first used to vaccinate infants and children. By 1949, it was officially licensed.
The DTP vaccine would herald a new era of vaccination—one in which combination vaccines became a cornerstone of pediatric and adult immunization programs.
When immunization schedules saw dramatic increases in the required number of vaccines, a parent could watch their child be injected as many as six times in a single pediatric visit in order to comply with the recommendations. The high number of shots was traumatizing for children, not to mention for their parents, making them less than eager to return to the pediatrician’s office.
Thus, reducing the number of injections by combining vaccines was sold by the vaccine industry as a way to reduce trauma and thus boost rates of compliance. Combination vaccines such as DTP were also sold as an opportunity to incorporate new vaccines into the schedule.
Along with ushering in a new era of amalgamated shots, the DTP vaccines popularized the use of mercury (thimerosal) in vaccines. Thimerosal was used ostensibly to “inactivate” whole cells in vaccines. It was marketed as a preservative that would prevent the growth of infectious organisms such as bacteria and fungi in multi-dose vaccine vials—thus allowing for repeated puncture of the same vial with less fear of contamination.
As we now know, the problems with thimerosal in vaccines have been acute and are well-documented. Mercury is highly toxic. It is persistent—that is, it stays in the body. And it can change into methylmercury. Many recent studies have highlighted the adverse neurotoxic effects of mercury during fetal development, even at very low levels of exposure.
Following a mandated review of mercury-containing food and drugs in 1999, the American Academy of Pediatrics and the US Public Health Service issued a joint statement that urged “all government agencies to work rapidly toward reducing children’s exposure to mercury from all sources.” The statement recommended that thimerosal be removed from vaccines as soon as possible.
Thimerosal is still used in multi-dose vials of some flu vaccines in the US. No vaccines in the European Union currently contain thimerosal as a preservative.
Problems with the DTP Vaccine
Severe reactions to the whole cell pertussis vaccine were observed as early as 1933. Going back to 1947, there were published reports of irreversible brain damage following injections of the whole-cell pertussis vaccine.
In 1948, the journal Pediatrics published an article documenting cases of brain damage that occurred following injections of the pertussis vaccine:
“Inspection of the records of the Children’s Hospital for the past ten years has disclosed 15 instances in which children developed acute cerebral symptoms within a period of hours after the administration of pertussis vaccine. The children varied between 5 and 18 months in age and, in so far as it is possible to judge children of this age range, were developing normally according to histories supplied by their parents. None had had convulsions previously.”
In 1958, the British Medical Journal (BMJ) issued a report titled “Neurological Complications of Pertussis Immunization, “which chronicled multiple serious adverse events associated with the pertussis vaccine. The authors examined 107 cases of injury and recorded the type of vaccine used for 68 of the cases. Of the 68 cases, 28 adverse events resulted from the pertussis vaccine alone, 12 were from the combined diphtheria-pertussis vaccine, and 28 came from the combined diphtheria-tetanus-pertussis vaccine.
The authors concluded that their findings reflected trends in vaccination, that no significant difference in outcome occurs whether the pertussis vaccine is used alone or in combination with other vaccines, and that “it is generally agreed that the pertussis antigen in all these vaccines is responsible for the reported neurological sequelae.”
Beyond those instances of severe neurological damage, the BMJ study also found that “in 8 cases death was reported within 48 hours of inoculation, 7 of them within 24 hours.”
By the 1960s, it was acknowledged within the medical community that the pertussis in the DTP vaccine presented a known risk of convulsions and brain damage in children.
A 1960 article published in the BMJ and written by Justus Ström, MD, who worked in a hospital for infectious diseases in Stockholm, Sweden, questioned the justification for this vaccine.
Considering the observed neurological complications after the pertussis combination vaccine, Dr. Ström remarked:
“The increasingly mild nature of whooping-cough and the very low mortality in this disease in Sweden makes it questionable whether universal vaccination against it is justified. The same question may perhaps arise in some other countries.”
By 1979—almost two decades after Dr. Ström’s article—Sweden discontinued use of the whole-cell pertussis vaccine.
Meanwhile, the same question he posed was being routinely and roundly ignored in the United States.
On March 9, 1979, the Tennessee State Department of Health reported to the CDC that four infant deaths had occurred within twenty-four hours of receiving the DTP vaccine. A government investigation of the sudden infant deaths (SIDS) in Tennessee showed that in 1978-79, eleven babies were found to have died within eight days of a DPT vaccination. Nine of the eleven had been vaccinated with the same lot of pertussis vaccine, manufactured by Wyeth Laboratories (acquired by Pfizer in 2009), and altogether five had died within twenty-four hours of vaccination.
A 1979 internal Wyeth memo revealed the baked-in cynicism of the pharma company’s representatives. Rather than address safety concerns, they spoke of the need for “limiting the distribution of a large number of vials from a single lot to a single state, county or public health department.”
Clearly this strategy of breaking up and distributing a single lot to various jurisdictions seeks to obfuscate the connection between the DTP vaccine and SIDS-related deaths by limiting the numbers of adverse events showing up in the same place at the same time. This practice is still used by all pharmaceutical companies today.
The timing of so many SIDS deaths in Tennessee made it impossible to deny the vaccines that the vaccines were at fault. At first, the FDA heeded the political pressure to recall the lots. But the regulatory agency caved under industry pressure and reversed course, allowing the dangerous lots to continue to be used.
In 1976, the UK government commissioned the National Childhood Encephalopathy Study, largely as a result of rising public concern about the safety of pertussis vaccines. The three-year study was released in 1979, coinciding with the SIDS cases in Tennessee. It showed “a significant association between serious neurological illness and pertussis vaccine.”
By the early 1980s, public awareness of problems associated with the DTP vaccine was reaching a fever pitch. The lawsuits alleging damage caused by the vaccine were growing.
In 1982, after a year of research, investigative journalist Lea Thompson revealed her findings in the nationally aired television documentary DPT: Vaccine Roulette.
This award-winning program was followed in 1985 by the book DPT: A Shot in the Dark: Why the ‘P’ in the DPT Vaccine May Be Hazardous to Your Child’s Health, authored by Harris L. Coulter and National Vaccine Information Center (NVIC) co-founder Barbara Loe Fisher. Their book is said to have sparked the first modern popular concern about the risk of neurological damage from a vaccine.
Thompson’s documentary and the Fisher/Coulter book vividly recounted multiple first-hand experiences of parents who watched their babies suffer severe and permanent DPT vaccine reactions.
It would not be an exaggeration to say that reactions to the anti-DTP documentary and book, in combination with an onslaught of costly lawsuits, catalyzed what is arguably the single most important—and controversial—piece of legislation in medical history.
The confluence of these events resulted in the establishment of the National Childhood Vaccine Injury Act of 1986. (A full report on the 1986 Act will be part of HFDF’s series on vaccines.) The NCVIA was signed into law, as part of a larger health bill, by United States President Ronald Reagan on November 14, 1986.
When President Reagan asked Wyeth why the company couldn’t make safer vaccines, Wyeth said the reason is because vaccines are “unavoidably unsafe.”
A year before the 1986 Act’s passage, a US Senate committee with oversight on public health convened in 1985 to discuss how to handle the flood of lawsuits being brought by Americans injured by vaccines.
The number of cases and the cost of the lawsuits arising from encephalitis (brain inflammation), encephalopathy (brain injury), and death from the DPT shot became so large that manufacturers were faced with problems obtaining liability insurance—and some were exiting the business. Average amounts claimed per suit rose from $10 million to $46.5 million between 1978 and 1984, leaving Lederle Laboratories as the only manufacturer of the pertussis vaccine by 1985.
The NCVIA would result in pharmaceutical companies receiving full liability protection for injuries resulting from childhood vaccines—”no matter how toxic the ingredients, how negligent the manufacturer or how grievous the harm.” This protection from financial liability was sold to the public as a way “to ensure a stable market supply of vaccines, and to provide cost-effective arbitration for vaccine injury claims.”
Under the NCVIA, the National Vaccine Injury Compensation Program (NVICP) was created to provide a federal no-fault system for compensating vaccine-related injuries or death by establishing a claim procedure involving the United States Court of Federal Claims and “special masters” who oversee the claims.
Since 1986, with no liability, thus no lawsuits, hence no huge losses hurting the bottom line, vaccine industry revenues have skyrocketed from $1 billion to $44 billion as of 2018.
DTaP Replaces DTP
“My own view, based upon some years of observation and experience, is quite firm. I supported the use of the vaccine in 1951 and subsequently with very little hesitation until about 1972, and gave pertussis vaccine between 1951 and 1956 to each of my four children. I would not dream of doing so again because it has become clear to me not only that the vaccine is incompletely protective, but also that the side-effects which I thought to be temporary are in fact dangerous, unpredictably so. There is no doubt in my mind that in the UK alone some hundreds, if not thousands, of well infants have suffered irreparable brain damage needlessly and that their lives and those of their parents have been wrecked in consequence.” — Professor Gordon Stewart (1980)
The DTaP vaccine is a direct descendant of the DTP vaccine.
Acellular pertussis vaccines (DTaP) were first developed in Japan in the 1970s as a response to adverse events arising from the whole cell vaccine. From those DTP adverse events there emerged in the mid-1970s an anti-vaccine movement. DTaP vaccines were introduced into the Japanese population in 1981.
Meanwhile, the CDC, faced with widespread misgivings surrounding DTP safety, recommended that the whole cell pertussis vaccine be phased out and replaced by acellular inoculation. As in Japan and other developed nations, the vaccine industry in the US was forced to shift production towards the acellular vaccine, considered less reactogenic though it was more expensive.
But while developed countries halted the use of DTP in their populations, international organizations continued to promote and distribute the DTP vaccine to developing and underdeveloped countries.
The DTaP vaccine was first licensed in the US in 1991. The premise was that the acellular vaccine was safer and would produce fewer side effects and deaths than the whole cell version of the vaccine. At this time, there were two versions (Tripedia and ACEL-IMUNE) and multiple producers of the acellular vaccine.
In 1997, the Advisory Committee on Immunization Practices (ACIP) finally recommended that the whole cell pertussis vaccine be replaced by the acellular vaccine for all five doses in the series. ACIP claimed that DTaP vaccines would be “less likely to provoke adverse events because they contain purified antigenic components of Bordetella pertussis.”
At the same time, ACIP frankly admitted, “Concerns about safety [of DTP vaccines] prompted the development of more purified (acellular) pertussis vaccines that are associated with a lower frequency of adverse events and are effective in preventing pertussis disease.”
The CDC’s 1997 Morbidity and Mortality Weekly Report (MMWR) on the ACIP recommendation was fraught with so many inconsistencies and contradictions that a careful reader might question the credibility of the CDC and its assessment of the value of DTP/DTaP vaccines.
For example, the report states that in the US “the highest recorded annual incidence of pertussis occurred in 1934 when greater than 260,000 cases were reported.” It also noted that cases of pertussis declined by 99% from 1934 to 1970, when the “use of whole-cell DTP vaccines became widespread.”
What the report fails to mention, however, is that most of that decline came before the introduction of the vaccine in the late 1940s. The exact same trend happened with other diseases, such as scarlet fever, measles, tuberculosis, and typhoid.
The 1997 report then confesses that an “increase in reported pertussis cases has occurred despite pertussis vaccination coverage levels that are higher (93% by 1994) than at any time in the past.” Its authors go on to highlight the inadequacies of the DTP injection, using their own metrics.
How can a claim that the DTP injection was responsible for a reduction in whooping cough cases be believed, when the evidence clearly shows that over 90% of the reductions in this disease occurred before the vaccine was in use? And how can the CDC justify its support for the vaccine when the same report concedes that the pertussis cases are high despite near universal vaccine rates?
In the same time period, randomized controlled trials in Sweden and Italy were also showing low clinical efficacy for the whole cell DTP vaccines.
Blind to its own contradictory statements, the CDC soldiered on, asserting that “the current pertussis vaccination program in the United States, which has relied on four different whole-cell DTP vaccines for primary vaccination, remains high.”
Given the profound historical problems and incongruities with the DTP program, it’s fair to ask: Can we believe the same industry and agencies when they tell us the DTaP vaccine is safer?
The answer to that question starts with a look at the design of the clinical trial data for two of the mainstays of the DTaP program, DAPTACEL and INFANRIX.
As is common practice in the vaccine industry, there was no valid placebo used in the DAPTACEL and INFANRIX clinical trials—just as there was no placebo in the trials of the earliest acellular vaccines such as Tripedia.
A close look at the package insert for GlaxoSmithKline’s (GSK) INFANRIX reveals vaccine trials in which the DTP vaccine is used as a control. Not only was the DTP vaccine never licensed in a placebo-controlled trial, but it is widely considered one of the most reactive vaccines in the history of the industry. To put it bluntly, DTP is a vaccine known to increase mortality.
Simple logic dictates that claiming a product is safe when compared to one of the most dangerous products ever manufactured by an industry is not a legitimate measurement of that product’s safety.
The DAPTACEL trial design was as duplicitous as the INFANRIX trials. It, too, used the DT or DTP vaccine as the control.
Other problems with the studies done on those vaccines included the use of unequal numbers of subjects between groups and the non-random selection of subjects.
To illustrate: In one DAPTACEL study, the children included in the fifth dose studies were non-random subsets of participants from previous DAPTACEL or Pentacel studies. We must ask: Why were these subsets selectively chosen and how was that selection process determined? We do not have the answer to either question.
Another significant shortcoming of those trials was the consistent rate of attrition (children who dropped out or were removed from the study), which resulted in fewer participants as the studies progressed.
In one three-dose trial study for INFANRIX, the number of participants went from 335 for the first dose down to 315 by the third dose.
In a larger, four-dose DAPTACEL study, the number of subjects went from 1,406 for the first dose down to 1,144 by the fourth dose.
No explanation was given in the manufacturer’s inserts as to why the children were removed from the study. Is it possible that a parent might pull a child from the trial if the child suffers an adverse reaction? There is some evidence that points in this direction. We know that the percentage of children who experienced systemic reactions during the trials increased with each successive vaccine in the series. If this were the reason for the rates of attrition, the results of those trials were seriously compromised.
But it wasn’t only the flawed trial designs that raised red flags about the validity of claims that the TDaP vaccine was a “safer and more effective” alternative to the whole cell DTP vaccines.
This claim has been called into question for a number of other reasons.
The Failure of DTaP
In 2012, Danish researchers published the study “Risk of Febrile Seizures and Epilepsy After Vaccination With Diphtheria, Tetanus, Acellular Pertussis, Inactivated Poliovirus, and Haemophilus Influenzae Type b.,” which found children eight times more likely to have a febrile seizure on the day of vaccination of DTaP-IPV-HiB vaccine than on any non-vaccinated day.
The Danish study cited a previous study conducted in the UK, which examined the risk of seizures after DTaP-IPV-Hib vaccinations given at 2 months, 3 months, and 4 months “found a 2-fold higher risk of seizures on the day of vaccination.”
Also, the Danish study referred to a study from the United States that examined the risk of seizures after DTaP vaccination given at 2, 4, 6, and 15 to 18 months “found a 30% higher risk of seizures on the day of the first DTaP vaccination.”
A 25-year compilation of data from the CDC on the various iterations of the TDaP injections reported a significant number of adverse reactions associated with the TDaP vaccine:
“Roughly one in nine (11.2%) of the reported AEs coded as serious, and 15% of all serious AEs were deaths (844/5,627).”
What about DTaP vaccinations in relation to what are called “whooping cough outbreaks”? How can these outbreaks be explained if the vaccine is effective and if vaccination rates for pertussis in the US are at 95%?
A 2016 study, “Waning TDaP Effectiveness in Adolescents,” published in Pediatrics, looked at 1,200 California adolescents who received TDaP during pertussis outbreaks between 2010 and 2014, found that “vaccine effectiveness” among them declined to less than 9% in four years, and concluded:
“Routine TDaP did not prevent pertussis outbreaks. Among adolescents who have only received DTaP vaccines in childhood, TDaP provided moderate protection against pertussis during the first year and then waned rapidly so that little protection remained 2-3 years after vaccination.”
Another study, “Sources of Infant Pertussis Infection in the United States,” published in Pediatrics in 2016, concluded:
“Despite high or increasing coverage with pertussis vaccines, the incidence of disease has been slowly increasing in a number of countries, with notable epidemic peaks in recent years”, and that most pertussis cases in the US were occurring among “recently vaccinated children and adolescents.”
Two other case studies add to the literature illustrating the failure of the pertussis vaccine.
One took place in February 2019, when Harvard-Westlake, a private high school in Los Angeles, reported a pertussis outbreak that impacted 30 students. School spokesperson Ari Engelberg noted that the school had a “really high vaccination rate” and that the 30 students who developed pertussis were all vaccinated. Out of approximately 1,600 students, only 18 had medical exemptions allowing them to opt out of vaccinations, and “none of those 18 students contracted the disease.”
The other was in December of the same year, when a whooping cough outbreak forced the closure of St. Theresa Catholic School and Daycare Center in Houston, Texas. The school noted that its students were 100 percent vaccinated. “Doctors are unsure why vaccinated children may still get the disease,” noted its pastor. Public health officials suggested the likelihood of “waning immunity.”
Parallel to these case studies, Pediatrics published the 2019 Kaiser Permanente California study “Acellular Pertussis Vaccine Effectiveness Over Time.” It tracked 469,982 children between 1999 and 2016 and identified 738 pertussis cases.
The study showed that 82% of pertussis cases were in children who had been fully vaccinated and an additional 5% occurred in partially pertussis-vaccinated children. The study also showed that for children 1-to-7 years old the risk of contracting pertussis increased fivefold three or more years after vaccination.
Employing Mad Hatter logic, the study concluded:
“Undervaccinated and especially unvaccinated children were at greater risk of pertussis. However, most pertussis cases occurred among children age-appropriately vaccinated who were further away from their last DTaP dose, suggesting that suboptimal vaccine effectiveness played a major role in recent pertussis epidemics.”
A 2013 infant baboon study published in The Proceedings of the National Academy of Sciences (PNAS), a peer-reviewed journal of the National Academy of Sciences (NAS), provided strong evidence that the whole cell and acellular pertussis vaccines are incapable of preventing infection or transmission of the alleged infection.
The study’s scientists found that whether baboons were vaccinated with whole cell (wP) or acellular vaccines (aP), by their measure the baboons were all capable of contracting pertussis and allegedly transmitting the illness to other baboons.
Citing two additional studies (here, and here), the authors noted:
“[R]ecent cohort and case-control studies concluded that following the fifth aP dose, children are fourfold to 15-fold more likely to acquire pertussis compared with within the first year, consistent with waning aP immunity.”
Researchers from the Center for Infectious Disease Dynamics (CIDD) at Penn State University showed that “acellular pertussis vaccination enhances B. parapertussis colonization.” Their 2018 study discovered a forty-fold enhancement of B. parapertussis colonization in the lungs of vaccinated mice, leading the authors to conclude that “the vaccine may be contributing to the observed rise in whooping cough incidence over the last decade by promoting B. parapertussis infection.”
So, what’s going on? No matter how high the vaccination rates, there appear to be consistent reports of pertussis cases and whooping cough outbreaks.
A 2016 review titled “The Pertussis resurgence: putting together the pieces of the puzzle” demonstrates that the pertussis vaccine is ineffective and that children receiving the vaccine have a much higher risk of getting whooping cough than do unvaccinated children.
One reason offered is that “toxins in Bordetella pertussis bacteria stimulate the production of large amounts of thick, sticky mucus that can clog the airways of tiny babies and children, making it difficult for them to take a breath as they struggle to breathe.”
Another reason offered is that a different Bordetella strain, parapertussis, can also cause whooping cough, though it is largely ignored in the current treatment regimen. This hypothesis relies on the notion that, since nature abhors a vacuum, when one Bordetella strain is suppressed, the void is filled by another strain.
Of course, such theories are based on the presupposition that these bacteria exist in the first place.
Legendary toxicology researcher and author Jim West suggests that the pertussis bacterial paradigm that Jules Bordet “declared” in 1906 (described in the beginning of this article) is not adequate to explain the causes for this disease.
In a paper titled “Whooping Cough and Pesticide Programs California Counties 2010,” West showed that the 2010 pertussis epidemic in California correlated with pesticide spray programs and peak outdoor pollution in the area. Based on that analysis West postulated:
“Pertussis is a seasonal pesticide disease, although it possibly can occur any time due to indoor air pollution, such as stove exhaust, indoor pesticide application, etc., or other sources. Pertussis could also occur due to outdoor air pollution, mining, refineries, vehicular, etc. Pertussis is a respiratory disease, and its symptoms are the body’s attempts to avoid breathing polluted air.”
Elsewhere, West made a similar argument suggesting petrochemical pollution was the causative agent for the whooping cough epidemic that hit Colorado in 2012.
Summary
“I also discovered how science can go horribly wrong. We can easily become captured by a belief system that is built on a shaky and flawed foundation. How often do we believe in something, not because we have done in-depth research on it, but because authority figures tell us it is the truth? What if what we believe is just an illusion?” ― Suzanne Humphries, Dissolving Illusions
The understanding that disease is the inevitable outcome of toxic environments, of food scarcity, and of unsanitary living conditions has existed since ancient times. In modern times, various social, political, and economic reforms have helped to eradicate or mitigate most of what are considered “infectious diseases” in the developed world.
“Modern medicine” has taken credit for the eradication of certain diseases over the past 100 years, when in fact recognition for these public health achievements properly belongs to those individuals whose contributions have led to improvements in sanitation, refrigeration, water works, labor laws, nutrition, and social services and social systems.
Given that the odds of contracting—much less being seriously impacted by—the three diseases covered in this article are extremely low, and given that the valid concerns over the safety of the three-in-one vaccines covered in this article are well documented, what could possibly be the logical rationale for keeping these risky medical products on the market?
One obvious answer is contained in this remark made by Robert F. Kennedy Jr. during his October 2020 London Real interview (scroll to the 14:45-minute mark):
“It’s like the dream product. If you could get your vaccine onto the CDC’s mandatory schedule, it’s worth about a billion dollars in profit to your company every year for every vaccine you can get on[to] that schedule.”
Those windfall profits from this “dream product” come with virtually no financial or legal liability for the pharma companies, thanks to the National Childhood Vaccine Injury Act.
Another justification for continuing to market diphtheria-tetanus-pertussis could be that if this combined vaccine is ever acknowledged by the medical establishment as unsafe, ineffective, and unnecessary, parents might begin to question other vaccines and, ultimately, the entire childhood vaccination program.
As Barbara Loe Fischer says in the last sentence of her 1985 book, DPT: A Shot in the Dark:
“The time has come to become educated about vaccines.”
===
The next installment, Part 4 of our 14-part series, examines the Haemophilus influenzae type b (Hib) vaccine.












